The heat produced by concrete during curing is called heat of hydration. This exothermic reaction occurs when water and cement react. The amount of heat produced during the reaction is largely related to the composition and fineness of the cement.
Monitoring the temperature of your concrete pour after placement according to ASTM guidelines is one of the most important steps in the construction of a concrete structure. That is why ensuring optimal curing conditions for your element is critical, especially during extreme weather conditions. The hydration process can be drastically impacted if freshly placed concrete is exposed to temperatures that are too high or too low, compromising the strength development of a mix design. Furthermore, if the temperature differentials are too high, thermal cracking can occur. By closely monitoring temperature variances in your concrete element during curing you will ensure that the strength, quality, and durability of your structure is acceptable.
The heat produced by concrete during curing is called heat of hydration. This exothermic reaction occurs when water and cement react. The amount of heat produced during the reaction is largely related to the composition (Table 1) and fineness of the cement.
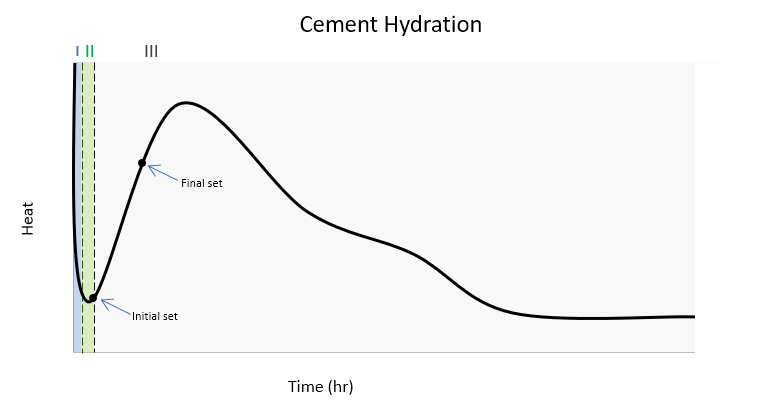
The Five Phases of Heat Evolution in Concrete
Heat evolution in concrete is a very complex and extensively researched topic. To simplify this process, heat evolution over time can be separated into five distinct phases. The heat profile can change depending on the type of cement. Typical hydration for Type I cement is graphically represented in the figure below.
| Portland Cement Phases | Abbreviation (Chemical Formula) |
|---|---|
| Dicalcium silicate | C2S |
| Tricalcium silicate | C3S |
| Tricalcium aluminate | C3A |
| Tetracalcium aluminoferrite | C4AF |
| Calcium sulphate * | CaSO4, CaSO4·2H2O (gypsum), CaSO4·½H2O |
…
